In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. The other two valence electrons of the Mg 2 Si formula unit are distributed equally between these ions. The ionic radii found in this study lie between Pauling’s ionic radii calculated for Mg 2+ and Si 4− and the covalent radii, and are thus consistent with the calculated covalent contribution to the bonding. Electronegativity of Magnesium is 1.31.
How many valence electrons are in an atom of magnesium?
2 Answers
Magnesium has two valence electrons.
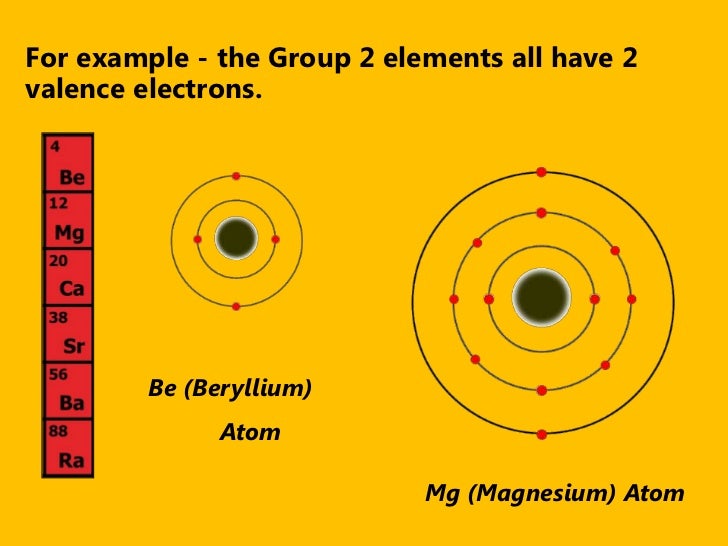
Magnesium has two valence electrons. Magnesium is element 12 and belongs to Group 2 of the Periodic Table. An element in Group 2 has two valence electrons. Also, the electron configuration of Mg is 1s² 2s²2p⁶ 3s² or Ne3s². Magnesium needs access to six valence electrons to satisfy the octet rule. Magnesium can remove six electrons from oxygen, forming ions and leading to an ionic bond. Oxygen needs access to two electrons to satisfy the octet rule. Magnesium prefers to lose two valence electrons to satisfy the octet rule.
Magnesium is element 12 and belongs to Group 2 of the Periodic Table. An element in Group 2 has two valence electrons.
Also, the electron configuration of Mg is 1s² 2s²2p⁶ 3s² or [Ne]3s². Since the 3s² electrons are the outermost electrons, magnesium has two valence electrons.
Valence Electrons In Magnesium
Explanation:
The electron configuration for magnesium is shown below.
Valence Electrons Calculator

The valence elctrons are the electrons in the third energy level (3s sublevel has two electrons in an atom of Mg)
Here is a video which discusses how to determine the electron configuration of magnesium.
Valence Electrons In Magnesium Is 2
Related questions
