Atomic mass of aluminium is 27 u and atomic number is 13, find the number of protons and number of neutrons in aluminium.
Aluminum is an abundant, light, and strong metal which has found many uses. The main drawback to its use is the large amount of energy necessary to refine it from its common ore, bauxite. The recycling of aluminum scrap metal saves over 90% of the energy required to separate aluminum from bauxite.
Aluminum is only about one third as dense as iron, but some of its alloys, such as duraluminum are as strong as mild steel. Duraluminum is formed from 94.3% aluminum, 4% copper, 0.5% manganese, 0.5% magnesium, and 0.7% silicon. While much stronger than pure aluminum, this alloy is less resistant to corrosion and is often clad with pure aluminum.
Because of its lightness and strength, aluminum is used widely in aircraft construction. It has high electrical conductivity, 80% of that of copper, and is used in place of copper in large electrical conductors. Its drawback there is its tendency to oxidize at contacts, requiring the use of contacts coated with an antioxidant.
The gram atomic mass of aluminum, which is defined as the mass of Avogadro's Number of atoms of the element, is 26.9815. To the justified number of significant digits, this is the same as the gram. The atomic mass of aluminum is 26.981539 amu and the atomic number of aluminum is 13. This light, grey metal is able to resist corrosion which makes it ideal for many. Chat Now Send Inquiry It's Elemental - Isotopes of the Element Aluminum.

- One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Dividing 26.98 6.02 ×1023 yields a mass of 4.48 ×10−23 g for one aluminum atom.
- Atomic mass is an absolute mass, relative isotopic mass is a number without proportions and without units. To measure the number of atoms in a sample you will figure out how many moles the sample element contains. A mole is the choice of unit chemists. It’s equal to Avogadro’s number (6.02 X 1023) of atoms.
Aluminum is a very active metal, a fact often disguised by the fact that it rapidly forms barrier oxide layers on exposed surfaces, inhibiting its interaction. When strongly heated it burns rapidly in air, and in the form of a fine dust is explosive.
Aluminum is one of the big 8 elements in the Earth's crust, being the third most abundant element at about 8.1% by weight. It is a constituent of the class of silicates called feldspars, which are the most abundant minerals in the Earth's crust. It is also a constituent of mica. One aluminum silicate mineral is pyrophyllite, AlSi2O5(OH). Aluminum combines with other metals in many silicates, e.g., manganese in spessartine, Mn3Al2(SiO4)3. Some aluminum silicates, like zunyite , contain fluorine and chlorine. One aluminum silicate, Al2SiO4 (F,OH)2, forms the gem mineral topaz. A silicate with potassium , KAlSi3O8, forms the mineral microcline, which can be apple green to brown in color. A silicate with calcium, zoisite forms blue gems. The aluminum silicate kyanite forms blue crystals which can be of gem quality. Other lithium aluminum silicates are spodumene, LiAlSi2O9 and petalite, LiAlSi4O10.
Aluminum oxide, Al2O3, is called alumina and occurs in nature as the mineral corundum. Corundum and impure corundum called emery are used in making abrasive cloths and wheels. Pure corundum is colorless, but the addition of a small impurity of chromic oxide can make the precious stone ruby (red) and the addition of titanium oxide can make sapphire (blue and other colors). Another oxide of aluminum is diaspore, AlO(OH).
Aluminum along with magnesium form the oxide mineral spinel. Aluminum with zinc forms the oxide mineral gahnite. Aluminum with boron and fluorine form the oxide (borate) Jeremejevite.

One aluminum phosphate mineral is variscite, AlPO4.2H2O, which exhibits an apple green color and is popular as an ornamental stone. An aluminum phosphate with fluorine content is Wavellite. Another phosphate mineral of aluminum with sodium is Brazilianite, which can also be of gem quality. Aluminum appears with magnesium and iron in the phosphate lazulite . Phosphates of aluminum and iron are vauxite and paravauxite. A phosphate of aluminum and sodium is Wardite.
Aluminum sulfate is used in water purification. It is also used as a mordant in the dying of cloth. A mordant fixes the dye to the cloth, rendering it insoluble.
Aluminum has excellent reflective properties and is widely used in evaporated coatings for the manufacture of mirrors.
| Atomic data | Nuclear data |
How many protons and neutrons are there in Aluminium?
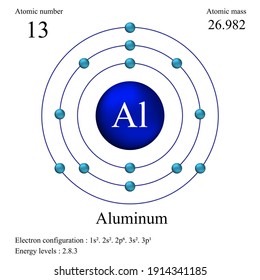
The atomic number, Z of aluminium, Al is 13. Hence there are 13 protons.
Note: Atomic number, Z represents the number of protons in a given atom.
Number of neutrons depends on the isotopic form of aluminium. More than 99.9% of naturally occurring aluminium is 13Al27.
Where 27 is the mass number, A; and 13 is atomic number, Z.
Now,
Mass number, A = no. of protons + no. of neutrons.
or
A = Z + no. of neutrons.
Therefore,
the number of neutrons in above isotope = A - Z = 27 - 13 = 14.
Note: The molar mass of aluminium, which is average of atomic masses of all isotopes = 26.981538 g/mol, since 13Al27 is the major isotope.
How do we know the number of neutrons in a given element?
Aluminum Number Of Protons
Actually, the neutrons in one isotope of an element can be known from the data. For most of the stable isotopes, this number is nearer to proton number. But not always. It is advisable to remember this data for some important elements.
Though it is meaningless to say that particular element has 'x' number of neutrons in it, however, if any one says like that, most of the time it refers to most abundant isotope. For example, you may have heard that hydrogen has no neutrons. In this case we are referring to the most stable and abundant isotope of hydrogen i.e., protium (1H1).
In the same way, we can also say aluminium has 14 neutrons and while doing so we should always keep in mind that we are citing 13Al27, the abundant isotopic form of aluminum.
Related questions
1) Write the electronic configuration of aluminium?
2) What is the chemical nature of aluminium?
3) What is the third most abundant element in the earth crust?
4) Write the formula of alumina?
Aluminum Atomic Mass Number
5) How many electrons are present in Al3+ ion?
Aluminum-26 Mass Number
6) Why aluminium dissolves in alkalies? Write the related chemical reaction.
7) What are the uses of aluminium?
Author: Aditya vardhan Vutturi